Treatment of isolated-perfused lungs with neuraminidase from Vibrio cholerae leads to pulmonary edema. A third key observation in our study is the pattern of alveolar edema observed when isolated-perfused lungs were treated with neuraminidase from Vibrio cholerae. A second key observation in our study is that terminally linked sialic acids are important contributors to endothelial barrier integrity. A principal observation in our study is that there is heterogeneity in sialic acid expression between endothelial cells of different vascular origins. Hepa-1c1c7 and PC-12 cells were used to study the binding of human properdin on the lesioned cell surfaces. The cell culture medium consisted of RPMI 1640 medium (Gibco), supplemented with 2 mM glutamine, 10% heat-inactivated horse serum, and 5% fetal bovine serum (all from Gibco). PC-12 cells grew as small clusters in suspension and were maintained in a density 5 × 105 cells/ml in culture flasks coated by 0.1% collagen type IV (Sigma-Aldrich). What is the market share of each type and application? Our research analysts will help you to get customized details for your report, which can be modified in terms of a specific region, application or any statistical details. Thus it will be of interest to determine if avian AAV also uses α2,3 sialic acid as its primary receptor.
 To confirm if both α2,3 and α2,6 sialic acids facilitate AAV1 and AAV6 transduction, we carried out a lectin competition assay on these three cell lines (Fig. If you liked this write-up and you would like to receive far more data regarding sialic acid manufacturer kindly go to our own web page. 5A to C). In addition, using a strain devoid of sialic acic transporter or by inactivation of endogenous sialic acic transporter gene, we have demonstrated that our living factory is capable of producing high level sialic acid in the culture media without the need of cell lysis. Indeed, Corfield and colleagues (4) demonstrated that linkage specificity by itself is not solely sufficient to determine the rate and extent of sialic acid hydrolysis by comparison of rates of sialic acid hydrolysis using several different glycolytic substrates. Expression cassettes can also be inserted into a host cell chromosome, using methods known to those of skill in the art. Other modification of the host cell described in detail below, can be performed to enhance production of the desired oligosaccharide. You can create a draft and submit it for review or request that a redirect be created, but consider checking the search results below to see whether the topic is already covered. See Paypal link on the VRM website (click on ‘Donate’ tab in upper right corner).
To confirm if both α2,3 and α2,6 sialic acids facilitate AAV1 and AAV6 transduction, we carried out a lectin competition assay on these three cell lines (Fig. If you liked this write-up and you would like to receive far more data regarding sialic acid manufacturer kindly go to our own web page. 5A to C). In addition, using a strain devoid of sialic acic transporter or by inactivation of endogenous sialic acic transporter gene, we have demonstrated that our living factory is capable of producing high level sialic acid in the culture media without the need of cell lysis. Indeed, Corfield and colleagues (4) demonstrated that linkage specificity by itself is not solely sufficient to determine the rate and extent of sialic acid hydrolysis by comparison of rates of sialic acid hydrolysis using several different glycolytic substrates. Expression cassettes can also be inserted into a host cell chromosome, using methods known to those of skill in the art. Other modification of the host cell described in detail below, can be performed to enhance production of the desired oligosaccharide. You can create a draft and submit it for review or request that a redirect be created, but consider checking the search results below to see whether the topic is already covered. See Paypal link on the VRM website (click on ‘Donate’ tab in upper right corner).
It also provides accurate information and cutting-edge analysis that is necessary to formulate an ideal business plan, and to define the right path for rapid growth for all involved industry players. Organizations, associations and alliances related to the Sialic Acid market industry. These data suggested that AAV1 and AAV6 use N-linked sialic acid for efficient transduction. These observations continue to add to our knowledge of AAV receptor/virus interactions and should provide important insight when determining optimum use of these reagents for vectors in human gene transfer studies. It is anticipated that human airway epithelial cells, the target cells for the treatment of cystic fibrosis, can be efficiently transduced by AAV1 and AAV6 vectors. In PAECs treated with neuraminidase from Clostridium perfringens, cells pulled apart from each other presumably through loss of cell-cell adhesions, whereas, in PMVECs treated with the same neuraminidase, the cells seemed to maintain most of the cell-cell interactions while losing cell-matrix interactions. C: PAECs (PA) and PMVECs (MV) were treated with 1 U/ml of neuraminidase from Clostridium perfringens. In these experiments we used a concentration of 1 U/ml of neuraminidase from Clostridium perfringens (Fig. 7C). The PAEC resistance rapidly decreased to ∼75% of baseline after addition of neuraminidase.
 Along those same lines, compared with one substrate that possessed α(2,3)-linked sialic acids (antifreeze glycoprotein 1-5) to another substrate that possessed (2,6)-linked sialic acids (α1-acid glycoprotein), neuraminidase from Vibrio cholerae hydrolyzed the (2,6)-linked sialic acids on the α1-acid glycoprotein faster than the α(2,3)-linked sialic acids on the antifreeze glycoprotein 1-5. Thus the molecular identity and structure of the protein (or lipid) and carbohydrate chains underlying the sialic acid moieties are also important in determining the availability and rate of sialic acid hydrolysis by neuraminidase enzymes. For example, we do not yet know whether α(2,6)- or α(2,3)-linked sialic acids, or both, are critically important for barrier integrity. More specifically, whereas PAECs surficially express both α(2,3)-linked and (2,6)-linked sialic acids, PMVECs principally express (2,3)-linked sialic acids. However, the fact that there was an actual increase in resistance suggests that there is something more going on, something we do not yet understand. To our surprise, the PMVEC resistance did not decrease at all following neuraminidase addition; in fact the resistance increased by ∼10%. Arrows denote time of neuraminidase addition. Over this time course, we observed a dose-dependent decrease in resistance.
Along those same lines, compared with one substrate that possessed α(2,3)-linked sialic acids (antifreeze glycoprotein 1-5) to another substrate that possessed (2,6)-linked sialic acids (α1-acid glycoprotein), neuraminidase from Vibrio cholerae hydrolyzed the (2,6)-linked sialic acids on the α1-acid glycoprotein faster than the α(2,3)-linked sialic acids on the antifreeze glycoprotein 1-5. Thus the molecular identity and structure of the protein (or lipid) and carbohydrate chains underlying the sialic acid moieties are also important in determining the availability and rate of sialic acid hydrolysis by neuraminidase enzymes. For example, we do not yet know whether α(2,6)- or α(2,3)-linked sialic acids, or both, are critically important for barrier integrity. More specifically, whereas PAECs surficially express both α(2,3)-linked and (2,6)-linked sialic acids, PMVECs principally express (2,3)-linked sialic acids. However, the fact that there was an actual increase in resistance suggests that there is something more going on, something we do not yet understand. To our surprise, the PMVEC resistance did not decrease at all following neuraminidase addition; in fact the resistance increased by ∼10%. Arrows denote time of neuraminidase addition. Over this time course, we observed a dose-dependent decrease in resistance.
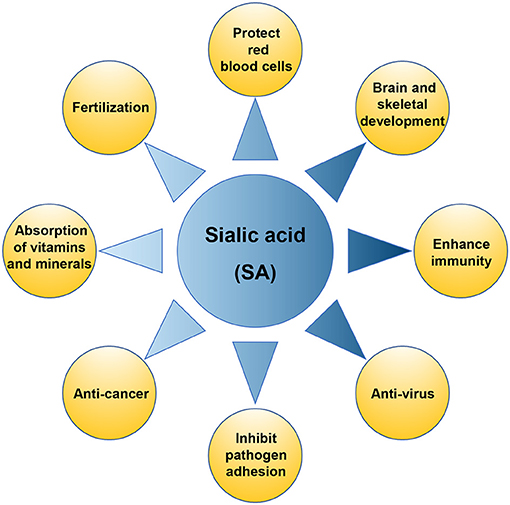

 The formula protein sources (whey vs casein) did not have a large impact on the ratios of free to bound sialic acids, nor did protein hydrolysis or sample form (solid vs liquid). Whole cell lysate (20 μl) was combined with 80 μl of 0.05 N H2SO4 (hydrolysis reagent) and incubated at 80°C for 60 min. If you have any kind of issues with regards to wherever and the best way to utilize
The formula protein sources (whey vs casein) did not have a large impact on the ratios of free to bound sialic acids, nor did protein hydrolysis or sample form (solid vs liquid). Whole cell lysate (20 μl) was combined with 80 μl of 0.05 N H2SO4 (hydrolysis reagent) and incubated at 80°C for 60 min. If you have any kind of issues with regards to wherever and the best way to utilize 

:max_bytes(150000):strip_icc()/The_University_of_California_1868.svg1-594c75c45f9b58f0fcef7f47.png) These models were then minimized, and we investigated whether the H4 atom of DANA was still able to transfer to nicotinamide. Using the high-resolution structure, we used a simple modeling approach to place a molecule of DANA a transition state
These models were then minimized, and we investigated whether the H4 atom of DANA was still able to transfer to nicotinamide. Using the high-resolution structure, we used a simple modeling approach to place a molecule of DANA a transition state